Whether or not those who are pregnant should take a Covid-19 vaccine to protect themselves and their infants became more controversial in May, when three top U.S. health officials – Health and Human Services (HHS) Secretary Robert F. Kennedy Jr., National Institutes of Health Director Jay Bhattacharya, and Food and Drug Administration director Marty Makary – announced that the Centers for Disease Control and Prevention (CDC) immunization schedule will no longer recommend it “for healthy children and healthy pregnant women.” The CDC web page featuring “General Recommendations for Vaccinating Pregnant Women” now says for Covid-19: “No guidance/not applicable.”
Past CDC studies found that women who are or recently were pregnant and contract Covid-19 are at greater risk of death than women who get Covid-19 and are not pregnant, and that vaccinating them provides some protection for their newborns.
Groups led by the American Academy of Pediatrics responded to the change with a lawsuit against the HHS secretary, arguing that his directive “is contrary to the wealth of data and peer-reviewed studies that demonstrate the safety and efficacy of Covid vaccines for children and pregnant women.” Other major medical associations also condemned the change.
“People who are pregnant can reduce the risks for themselves and their newborns by making sure that they are up to date on vaccinations before becoming pregnant and by taking the flu, Tdap, RSV, and Covid-19 vaccines during pregnancy on the schedule recommended by their health care provider,” said Annenberg Public Policy Center (APPC) Director Kathleen Hall Jamieson. “Even though those in charge of the CDC have withdrawn its support for Covid-19 vaccination during pregnancy, the science showing the value of that vaccination has not changed.”
Views about Covid-19 vaccination during pregnancy
Data from a new APPC health survey conducted August 5-18, 2025, among nearly 1,700 U.S. adults on a nationally representative panel, finds a reluctance among the public to recommend that someone who is pregnant get a Covid-19 vaccine, with just 38% saying they would do so. (Download the topline.)
Less than half of the survey respondents (42%) say it is safe to take an mRNA Covid-19 vaccine during pregnancy. And women who are between ages 18-49 years old, childbearing age, are even less likely than the rest of the adult population (men and older women) to say that mRNA Covid-19 vaccination during pregnancy is safe (36% vs. 45%).
In addition, women 18-49 are more likely (28%) than other adults (20%) to say it is false to state that Covid-19 vaccination during pregnancy is safe.
The percentage of women of childbearing age who think it is false to say the Covid-19 vaccine during pregnancy is safe has grown significantly since April 2024, from 19% then to 28% today.
Knowledge about the Covid-19 vaccine during pregnancy
Half of those surveyed know that Covid-19 vaccination minimizes the recipient’s chances of hospitalization and reduces the risk of complications from Covid-19:
- Protection against hospitalization with Covid-19: Over half (52%) correctly know that Covid-19 vaccination during pregnancy is effective at minimizing the chances of hospitalization with Covid-19, but 29% are not sure and 19% think the statement is false. Comparing women 18-49 years old with other adults, however, women of childbearing age are less likely to say the Covid vaccine protects against hospitalization: less than half (43%) of these women say it is true compared with 55% of the others, a significant difference.
- Reducing the risk of complications from Covid-19: Just over half (51%) of the public knows that getting a Covid-19 vaccine can reduce the risk of Covid-19 complications that can affect a pregnancy, but one-fifth (20%) think this is false and 30% are not sure. Knowledge of this outcome of the vaccine has declined significantly since April 2024, when 57% said it was true. There is no significant difference in the response between women 18-49 and other adults.
Flu, RSV, and Tdap vaccination during pregnancy
Though it no longer makes a recommendation about the Covid-19 vaccine during pregnancy, the CDC website continues to recommend that pregnant women get the flu, Tdap, and RSV vaccines.
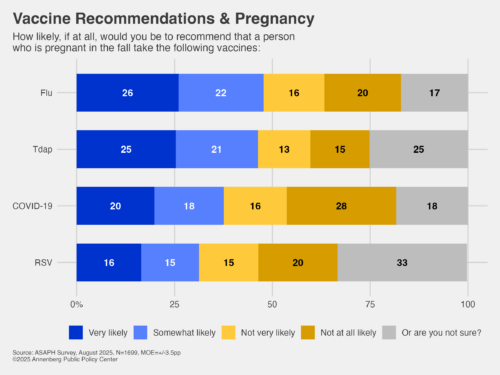
Yet less than half of those surveyed say they would recommend these three other vaccines to someone who is pregnant this fall:
- Flu: Less than half of respondents (48%) say they would be likely to recommend the flu shot this fall to someone who is pregnant if that person has not already received it this fall.
- Tdap: Under half (46%) say they would be likely to recommend the Tdap vaccine this fall to someone who is pregnant.
- Covid-19: Just 38% say they would be likely to recommend that someone who is pregnant this fall take the Covid-19 vaccine or booster if they are not up to date on their Covid-19 vaccinations. A larger group, 44%, say they are unlikely to recommend it.
- RSV vaccine: Just 31% say they are likely to recommend the RSV vaccine this fall during the 32nd to 36th week of pregnancy to someone who is pregnant, while a roughly equal group (35%) is unlikely to recommend it. The CDC says pregnant women should get a single dose of the maternal RSV vaccine during weeks 32-36 of pregnancy sometime between September through January.
Annenberg Science and Public Health survey
The survey data come from the 25th wave of a nationally representative panel of 1,699 U.S. adults conducted for the Annenberg Public Policy Center by SSRS, an independent market research company. Most have been empaneled since April 2021. To account for attrition, replenishment samples have been added over time using a random probability sampling design. The most recent replenishment, in September 2024, added 360 respondents to the sample. Wave 25 of the Annenberg Science and Public Health (ASAPH) survey, fielded August 5-18, 2025, has a margin of sampling error (MOE) of ± 3.5 percentage points at the 95% confidence level.
Download the topline and the methods report.
The policy center has been tracking the American public’s knowledge, beliefs, and behaviors regarding vaccination, Covid-19, flu, RSV, and other consequential health issues through this survey panel for four years. In addition to Jamieson, APPC’s ASAPH survey team includes research analysts Laura A. Gibson and Shawn Patterson Jr.; Patrick E. Jamieson, director of the Annenberg Health and Risk Communication Institute; and Ken Winneg, managing director of survey research.
See other recent Annenberg health survey news releases:
- Mosquito-borne illness: Despite increase in U.S. cases, worry about West Nile virus remains low (Sept. 26, 2025)
- Trust in health agencies and RFK Jr.: Public confidence in U.S. health agencies slides, fueled by declines among Democrats (Sept. 18, 2025)
- Vaccine mandates for school: Most Americans favor MMR vaccine requirements for public school (September 12, 2025)
- Health risks during pregnancy: Public knowledge high on smoking and alcohol risks during pregnancy (July 29, 2025)
- AI and health information: Many in U.S. consider AI-generated health information useful and reliable (July 14, 2025)
The Annenberg Public Policy Center was established in 1993 to educate the public and policy makers about communication’s role in advancing public understanding of political, science, and health issues at the local, state, and federal levels. Connect with us on Facebook, X, Instagram, and Bluesky.