How the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) determine whether a vaccine has resulted in the death of a vaccine recipient became the focus of renewed scrutiny by the press and public last month. In November 2025, one of the FDA’s chief vaccine regulators, Vinay Prasad, reported in a memo that although the FDA “never publicly admitted it,” children in the United States died as a result of COVID-19 vaccination.
 The public health community, press, and public will know more about how FDA researchers arrived at this conclusion when the sources and methods they used to tie COVID-19 vaccination to the deaths are revealed. What is known at this point is that the part of the vaccine safety system mentioned in the memo – the Vaccine Adverse Event Reporting System or VAERS – is a repository for unconfirmed reports that relies on voluntary submissions from individuals, families, healthcare professionals and others, and serves as a potential early-warning system. Other resources within the vaccination monitoring system increase researchers’ ability to determine whether the signal of a possible concern registered in VAERS or in one of the other sentinel entities reflects an actual concern.
The public health community, press, and public will know more about how FDA researchers arrived at this conclusion when the sources and methods they used to tie COVID-19 vaccination to the deaths are revealed. What is known at this point is that the part of the vaccine safety system mentioned in the memo – the Vaccine Adverse Event Reporting System or VAERS – is a repository for unconfirmed reports that relies on voluntary submissions from individuals, families, healthcare professionals and others, and serves as a potential early-warning system. Other resources within the vaccination monitoring system increase researchers’ ability to determine whether the signal of a possible concern registered in VAERS or in one of the other sentinel entities reflects an actual concern.
To facilitate understanding how the U.S. monitors vaccine safety, biostatistician Professor Jeffrey S. Morris, Ph.D., the George S. Pepper Professor of Public Health and Preventative Medicine at the Perelman School of Medicine of the University of Pennsylvania and a distinguished research fellow of the Annenberg Public Policy Center (APPC), authored the new APPC white paper, The Complementary Components of the U.S. Vaccine Safety Monitoring System. In the white paper, Morris analyzes the multicomponent U.S. system, including the passive surveillance system VAERS, the active surveillance Vaccine Safety Datalink (VSD) system, the FDA’s Sentinel Initiative and PRISM monitoring program, and V-Safe, a system developed by the CDC during the COVID-19 pandemic.
Morris offers a series of suggested improvements from pre-licensure clinical trials to the active and passive surveillance systems to communication about the system. “Although the COVID-19 pandemic drew greater public attention to vaccine safety, and to parts of the monitoring system, there is still limited understanding of the system’s full architecture, how each component operates, and how they are intended to interact to form an integrated safety net,” Morris writes. With this white paper, “The aim is to improve public explanations of the system, spur discussion on how to enhance it, and help sustain a robust safety monitoring infrastructure that earns public confidence.”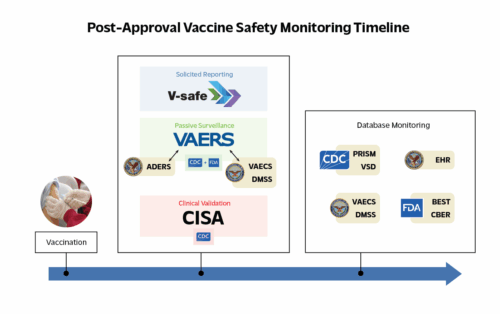 The Complementary Components of the U.S. Vaccine Safety Monitoring System is the third in the APPC vaccination communication toolkit. The earlier papers are:
The Complementary Components of the U.S. Vaccine Safety Monitoring System is the third in the APPC vaccination communication toolkit. The earlier papers are:


