Amid a surprisingly severe flu season and a Covid-19 resurgence, those highly contagious respiratory illnesses are drawing the largest share of media coverage and public attention. But it is also the season for another respiratory illness, respiratory syncytial virus or RSV, and RSV cases are “elevated in many areas of the country,” according to the Centers for Disease Control and Prevention (CDC).
RSV data for the week of Jan. 5, 2026, show an increase both in emergency department visits and hospital admissions for children up to age four, according to CIDRAP, the Center for Infectious Disease Research & Policy, at the University of Minnesota. And the Pan American Health Organization issued a Jan. 10 epidemiological alert warning RSV activity “is showing a gradual upward trend,” which, combined with flu cases, “could further strain health systems.”
RSV, a common respiratory illness, usually causes mild, cold-like symptoms, but is the leading cause of hospitalization among infants in the United States. Most RSV infections go away on their own, but RSV can be severe, especially for babies, young children, and older adults. The CDC estimates that more than 100,000 older Americans are hospitalized annually with RSV, as well as 58,000 or more infants and young children.
In mid-2023, U.S. health authorities approved two types of immunizations for RSV – vaccines for older adults and for pregnant people to protect their newborns; and a monoclonal antibody injection for newborns and infants.
In a nationally representative panel survey conducted Nov. 17-Dec. 1, 2025, the Annenberg Public Policy Center (APPC) of the University of Pennsylvania found an increase in awareness of immunizations that are available for RSV. The survey of 1,637 U.S. adults also found that about 6 in 10 respondents would recommend the vaccine or antibody injections to the groups recommended by the CDC, an increase from past years. See the topline for the data.
U.S. tightens guidance on children’s vaccines
The rises both in public familiarity with the RSV immunizations and recommending them come as U.S. health officials under longtime vaccine critic and Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. cut the number of routinely recommended childhood vaccinations and increased scrutiny of vaccines long-established as safe and effective.
Beginning in 2023, the CDC recommended RSV immunization for newborns and infants – either through a maternal RSV vaccine given to those who are pregnant during their 32nd-36th weeks or through a monoclonal antibody injection given to newborns and infants born during RSV season, typically October through March.
On Jan. 5, 2026, after this survey was conducted, U.S. health authorities cut the number of regular childhood vaccinations to 11 from 17. As a non-vaccine antibody product, RSV immunization is not part of this count, although health officials presented it as a tool that was also now being reserved for high-risk groups. An HHS spokesman, however, told the Washington Post that these high-risk groups include all otherwise healthy children whose mothers were not vaccinated against RSV during pregnancy. This effectively means the RSV guidelines did not change for newborns: Either those who are pregnant should be vaccinated during their pregnancy or their infants should get the monoclonal antibody injection.
The CDC also recommends the antibody injection for certain young children (ages 8 to 19 months) who are at increased risk of severe RSV disease.
Growing knowledge of vaccines against RSV
The CDC recommends RSV vaccination for adults 75 and older and people 50-74 years old who are at increased risk for severe RSV, if they did not get an RSV vaccine last year.
The survey finds different levels of familiarity with the immunizations for different groups, but all have increased from 2023 when the immunizations were first introduced:
The vaccine for older adults: The survey finds that a majority of adults (56%) are aware there is a U.S. Food and Drug Administration (FDA)-approved vaccine against RSV for older adults, up from 42% in October 2023, only months after CDC first recommended this treatment, in May 2023. In 2025, 39% are unsure whether there is a vaccine, compared with 53% unsure in October 2023.
The maternal vaccine to protect newborns: The survey also finds greater familiarity with the RSV vaccine for pregnant people, as compared with an August 2023 survey, when the vaccine first became available. In the current survey, 38% say there is an FDA-approved vaccine against RSV for pregnant people to protect their infants, up from 12% in August 2023. However, the percentage familiar with this vaccine was eight points higher in September 2024 at 46%, so the current figure represents a recent decline.
Increased likelihood of recommending RSV interventions
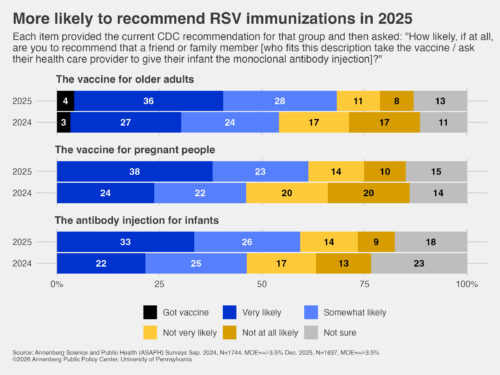
A majority of Americans say they would be likely to recommend the RSV vaccine for older adults and those who are pregnant and the monoclonal antibody injection for infants, as the CDC has recommended:
- Older adults: Nearly two-thirds (64%) say they would be likely to recommend the RSV vaccine to a friend or family member age 75 or older as well as those 50 to 74 years old with an increased risk of respiratory illness. That is higher than the 51% who would have recommended it to an older friend or family member in September 2024, 14 months earlier, when we asked the question with slightly different wording based on the then-recommended age guidelines. (See the topline for question wording.)
- During pregnancy: 61% say they would be likely to recommend the RSV vaccine to a friend or family member who is pregnant to protect their newborns. That is significantly higher than the 46% who would have recommended it to a pregnant friend or family member in September 2024, when this was asked with slightly different wording.
- Newborns: 59% say they would be likely to recommend the monoclonal antibody injection to parents of a newborn or infant born during RSV season (fall and winter) if their mother was not vaccinated against RSV during pregnancy, as recommended by the CDC. This is significantly higher than the 46% who would have recommended it in September 2024.
- Vaccines vs. antibody injections: By more than a 3-1 margin, respondents say they would be more likely to recommend that a pregnant friend or family member take an RSV vaccine to protect their infant (43%) than have the infant get a monoclonal antibody injection from a health care provider (13%). Nearly a third are not sure (30%) and 14% say they would do neither. This is statistically unchanged since September 2024. However, the preference for the RSV vaccine over the antibody injection has grown since October 2023, when fewer said they would choose the RSV vaccine during pregnancy (31%), 14% (no change) would recommend the monoclonal antibody, more were unsure (35%), and more said neither (20%).
“It’s encouraging to see the increase in the likelihood of recommending the RSV vaccine to people who are pregnant or the monoclonal antibody to parents of newborns and infants,” said Ken Winneg, managing director of survey research at APPC. “It’s important for OB-GYNs, pediatricians, and public health officials to communicate the value of these immunizations.”
Vaccine seen as safer than the disease
Most U.S. adults say that getting the RSV vaccine is safer than getting RSV:
- 70% say it’s safer for older adults to get the RSV vaccine than to get RSV – a five-point increase since February 2025, when 65% said it is safer for people over the age of 60 to get the vaccine than to get RSV.
- 58% say it’s safer for pregnant people to get the RSV vaccine than to get RSV – a five-point increase over February 2025, when 53% said it is safer for someone who is pregnant to get the vaccine than to get RSV.
Annenberg Science and Public Health survey
The survey data come from the 26th wave of a nationally representative panel of U.S. adults conducted for the Annenberg Public Policy Center by SSRS, an independent market research company. Wave 26 (n=1,637) of the Annenberg Science and Public Health Knowledge (ASAPH) survey was fielded Nov. 17-Dec. 1, 2025, and has a margin of sampling error (MOE) of ± 3.5 percentage points at the 95% confidence level. Figures are rounded to the nearest whole number and may not add to 100%. Combined subcategories may not add to totals in the topline and text due to rounding.
Download the topline and the methods reports.
The policy center has been tracking the American public’s knowledge, beliefs, and behaviors regarding vaccination, Covid-19, flu, RSV, and other consequential health issues through this survey panel since April 2021. APPC’s ASAPH survey team includes research analyst Laura A. Gibson; Patrick E. Jamieson, director of APPC’s Annenberg Health and Risk Communication Institute; and Ken Winneg, managing director of survey research.
See other recent Annenberg health survey news releases:
- Shared decision-making: CDC urges “shared decision-making” on some childhood vaccines; many unclear about what that means (Jan. 5, 2026)
- MMR vaccine safety: As measles cases rise, views of MMR vaccine safety and effectiveness and willingness to recommend it drop (Dec. 22, 2025)
- Whooping cough: Cases of whooping cough remain high, but knowledge about the disease still low (Dec. 18, 2025)
- Hepatitis B vaccine: Although public overwhelmingly supports hepatitis B vaccine for a newborn, partisan differences exist (Dec. 5, 2025)
- Vaccine safety: Americans more likely to trust American Medical Association than CDC on vaccination safety (Dec. 3, 2025)